
This cookie is set by GDPR Cookie Consent plugin. These cookies ensure basic functionalities and security features of the website, anonymously. Necessary cookies are absolutely essential for the website to function properly. The mass of a regular chlorine atom, Cl (35) is 34.968853 grams (per mole) and the mass of the isotope Cl (37) is 35.965903 grams (per mole).Ĭhlorine-35 (uncountable) (physics) The major stable isotope of chlorine, 35 17Cl, having seventeen protons and eighteen neutrons it amounts to about 76% of the element in nature. Obviously, the one with more neutrons will have more mass and this is confirmed in the table. What is the atomic mass of chlorine – 35? The atomic number of the element Chlorine is 17
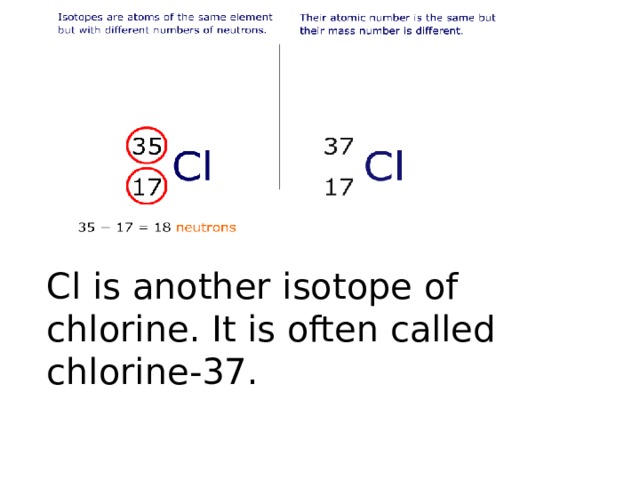
Chlorine – Protons – Neutrons – Electrons – Electron Configuration. Chlorine-35 is composed of 17 protons, 18 neutrons, and 17 electrons. How many protons neutrons and electrons are in chlorine 35?Ĭhlorine has two stable isotopes, 35Cl and 37Cl.
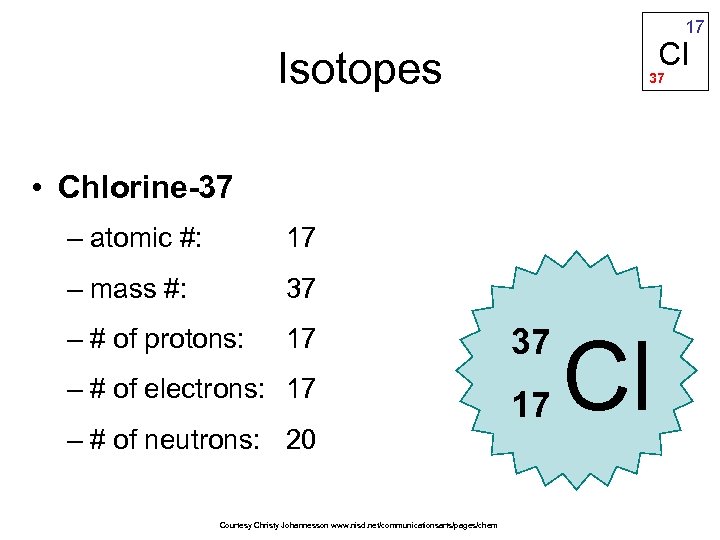
The longest-lived radioactive isotope is 36Cl, which has a half-life of 301,000 years….List of isotopes. There are two stable isotopes, 35Cl (75.77%) and 37Cl (24.23%), giving chlorine a standard atomic weight of 35.45. These differ only in physical contents and weights because neutrons contribute to the mass of an atom which is 35 and 37 in this case. In isotopes of 17Cl35 and 17Cl37 the atomic numbers are the same hence, their electronic configuration remains the same, and so are their chemical properties. Why do 35 17cl and 37 17cl have the same chemical properties? Chlorine is a chemical element having the atomic number 17 and chemical symbol Cl. The key difference between chlorine 35 and 37 is that chlorine 35 has 18 neutrons per atomic nuclei, whereas chlorine 37 has 20 neutrons per atomic nuclei. What properties will most likely be slightly different between chlorine-35 and chlorine-37? The number of protons an atom has, also known as the atom’s atomic number, determines which element it is. Why do chlorine-35 and 37 have the same chemical properties?Ĭhlorine-35 and chlorine-37 are not different elements because an atom of chlorine-35 and an atom of chlorine-37 each contain the same number of protons. The number 37 in chlorine indicates the mass number. The answer is 17 protons and 20 neutrons. What does the 37 indicate in chlorine-37? Chlorine-37 accounts for 24.23% of natural chlorine, chlorine-35 accounting for 75.77%, giving chlorine atoms in bulk an apparent atomic weight of 35.453(2) g/mol….Chlorine-37. Its nucleus contains 17 protons and 20 neutrons for a total of 37 nucleons. They both have the same atomic number(proton number) as they are the same element, hence they also have the same number of electrons. The numbers 35 and 37 are the mass numbers for the two isotopes of chlorine. Why does chlorine-35 and 37 have the same properties? What is the number of neutrons in chlorine-37?Ītoms and isotopes So although chlorine has a mass number of 35 which means it has 18 neutrons, it can also have a mass number of 37, which means it has 20 neutrons. Which statement is correct about chlorine-35 and chorine-37? They have the same number of protons and electrons, but a different number of neutrons.

Which statement is true about chlorine-35 and chlorine 37? Both Cl35 and Cl37 have same atomic number, hence the number of electron in both of these atoms will be same. Isotopes have same chemical properties but different physical properties. The atoms of same elements which have the same atomic numbers but different mass numbers are known as isotopes. Do CL-35 and Cl-37 have the same physical properties? Since chemical properties depends on electrons therefore isotopes have same chemical properties. 9 What is the atomic number of chlorine?ĭoes chlorine-35 and chlorine 37 have the same properties?Ĭl-35 and Cl-37 are isotopes i.e.8 How many protons neutrons and electrons are in chlorine 35?.6 Why do chlorine-35 and 37 have the same chemical properties?.5 What does the 37 indicate in chlorine-37?.3 Which statement is true about chlorine-35 and chlorine 37?.2 Do CL-35 and Cl-37 have the same physical properties?.1 Does chlorine-35 and chlorine 37 have the same properties?.


 0 kommentar(er)
0 kommentar(er)
